Coronavirus Vaccine Begins Clinical Trials
A coronavirus vaccine is in the final stage of clinical trials.
This article is more than 2 years old
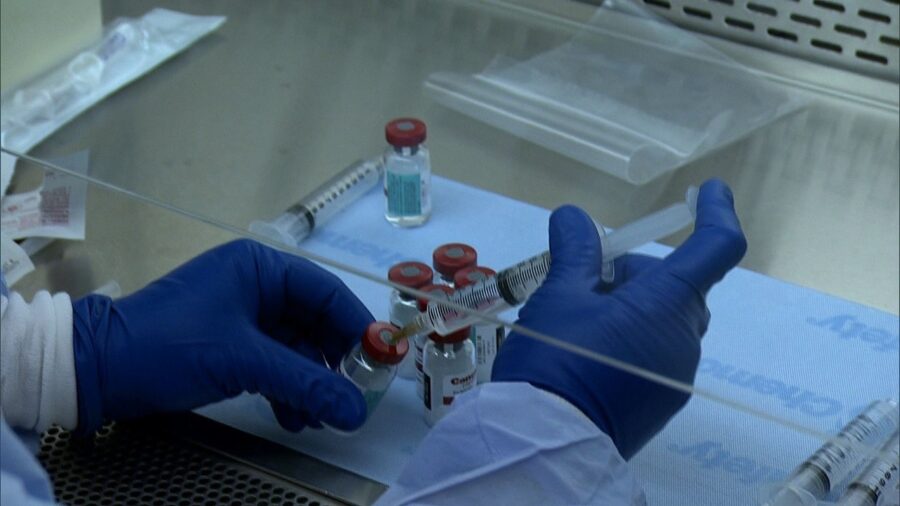
A coronavirus vaccine is in the final stages of clinical trials. Multinational corporation Johnson & Johnson is the fourth company to announce it is heading towards the last steps in creating and verifying a vaccine for the deadly virus. This comes after the United States has hit a devastating landmark with over 200,000 deaths being attributed to the coronavirus pandemic.
Though they are a couple of months behind other institutions in the race to develop a coronavirus vaccine, Johnson & Johnson have an advantage in that their advanced vaccine trial will constitute the largest attempt at proving a working vaccine. 60,000 participants will be involved in this final leg of the clinical trials. The company has stated that they will know if the vaccine works by the end of 2020.
There are other significant advantages to Johnson & Johnson’s potential coronavirus vaccine. This possible vaccine would not have to be kept frozen during transportation to hospitals and other delivery centers. If that is true, it would make the distribution of the vaccine much easier as it would not require refrigerated vehicles during transportation. The proposed Johnson & Johnson vaccine would also only require one shot instead of two. With this in effect, it would also help the mass production of a vaccine since everyone in the world needs to be vaccinated.
However, the political elements of a coronavirus vaccine are already rearing their ugly head. Donald Trump has repeatedly promised the American people that a vaccine will be ready and available before Election Day on November 3. The president has urged federal regulators to approve any potential vaccine as quickly as possible. This has led to an understandable concern that necessary vetting processes will be ignored. The pressure coming from the White House could end up hurting the vaccine’s development and review by scientific experts.
Thankfully, there are procedures being enacted that could help ensure proper vetting takes place in regards to a coronavirus vaccine. The F.D.A. is expected to announce stricter guidelines regarding clinical trial data, and Johnson & Johnson independently decided to release the detailed blueprints of their trials so that no accusations of corporate secrecy can be lobbed at them.
Even if Johnson & Johnson are able to get a coronavirus vaccine cleared by all the proper authorities, it is going to take more than one vaccine to innoculate the entire world populace. With seven billion people across the planet, no single manufacturer will be able to produce vaccines on that kind of scale. Other companies that are developing their own vaccine may need to work with Johnson & Johnson or move forward with their own vaccines.
We are in the middle of a historic moment. A vaccine has never been tested and mass-produced this quickly in all of human history. Normally, vaccine trials and manufacture can take years. This coronavirus vaccine would mark a turning point in the history of inoculation. Granted, we all want and need a vaccine as soon as possible. But, it is important that any and all vaccines be properly tested and vetted before being made widely available to the public. Hopefully, political aspirations will be set aside and the best science possible can be done.












